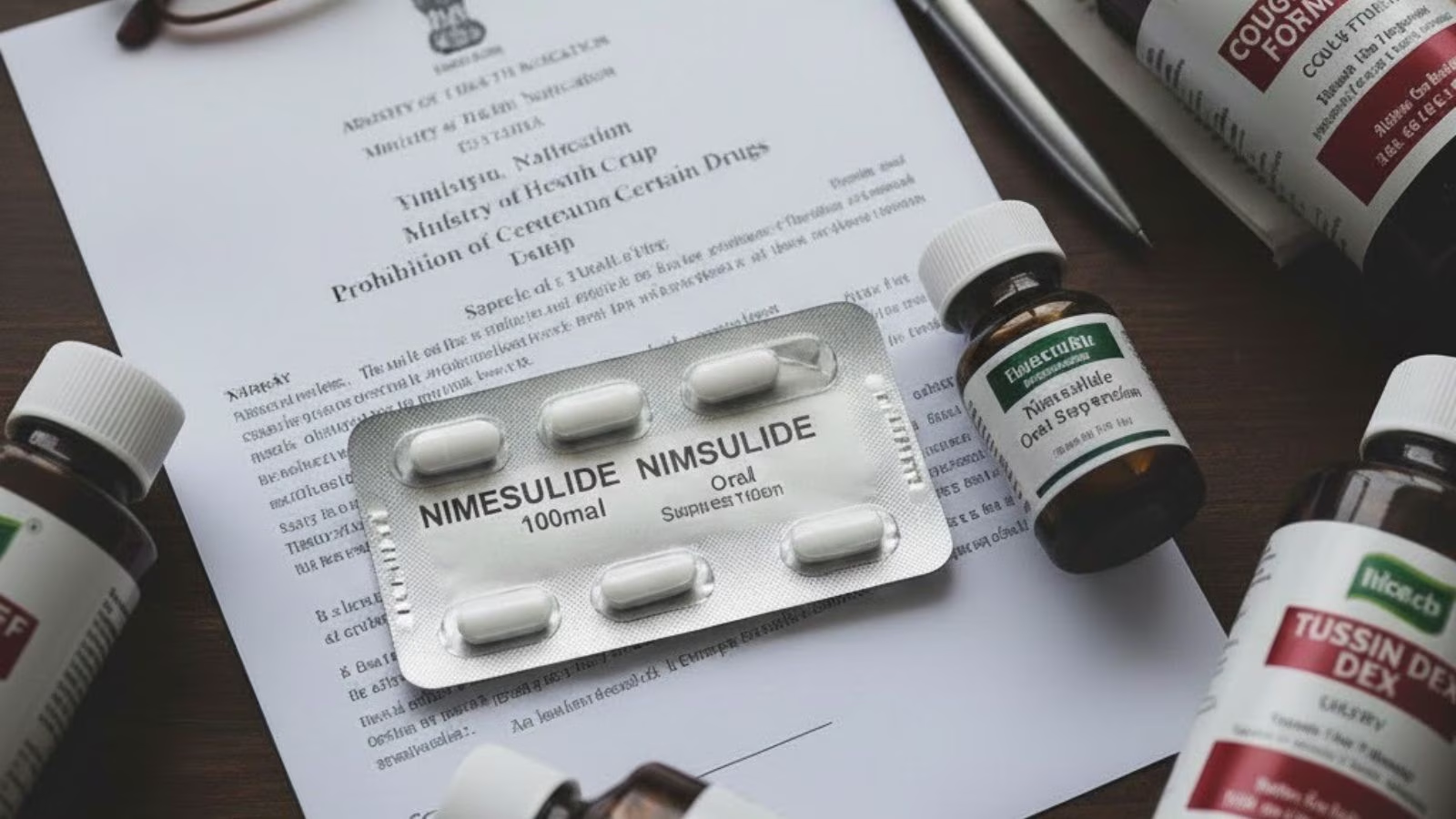
The government has banned the manufacture, sale and distribution of nimesulide, a common pain and fever medication, in all oral “immediate release” formulations above the dosage of 100 mg, saying it poses a health risk.
In a notification, the Ministry of Health and Family Welfare said the Central government is “satisfied that the use of all oral formulations containing Nimesulide above 100 mg in immediate release dosage form is likely to involve risk to human beings and that safer alternatives to the said drug are available”. It said it is “necessary and expedient in the public interest to prohibit the manufacture, sale and distribution of the said drug in the country for human use.” The notification specifically listed, “All oral formulations containing Nimesulide above 100 mg in immediate-release dosage form.”
Simultaneously, the Ministry also released a draft notification removing cough syrups from the list of over-the-counter medicines.
It removes “syrups … for cough” from Schedule K, the list of medicines exempted from the requirement of a prescription by a registered medical practitioner. Lozenges, pills or tablets for cough continue to remain on the list.
This comes in the wake of at least 22 children dying in Madhya Pradesh after consuming contaminated cough syrups. There have also been instances, including one in Rajasthan, where children given cough syrups not meant for them have died.
Earlier this year, an expert committee under the drug regulator said “DCC was apprised about the recent incidences due to contaminated cough syrup and it was proposed that the exemption provided… in respect of syrups for cough may be deleted.”
All stakeholders have been asked to submit their suggestions or objections over the next 30 days, after which the draft would be considered for implementation.
The banning of certain formulations of nimesulide was one of the recommendations made by the Indian Council of Medical Research after reviewing the effect of nimesulide on adults. This recommendation, along with other advice on prescribing the medicine, were accepted by an expert committee under the apex drug regulator. Nimesulide is known to cause liver toxicity in some cases.
The other recommendations by ICMR, which were accepted by the committee, included one that said that the drug be used only as a second line of treatment when other drugs fail to work or cannot be prescribed. The ICMR also recommended that the drug not be used in pregnant, lactating women and those planning for pregnancy.
It also recommended that nimesulide should not be prescribed to patients with kidney or liver impairment. It should also not be administered alongside other drugs that can be toxic to the liver or the kidneys. The drug is already banned for children under the age of 12.
The committee also asked ICMR to review the impact of the use of the drug by different ages – children below the age of 12, those between 12 and 18 years, and those above the age of 60.
For clarifications/queries, please contact Public Talk of India at:
+91-98119 03979 publictalkofindia@gmail.com

For clarifications/queries,
please contact Public Talk of India at:

